

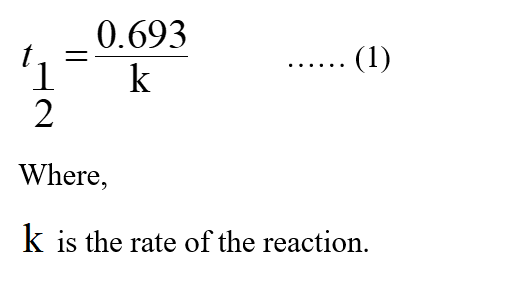
Second order – Successive half lives become shorter.Zero order – Successive half lives becomes longer.For example, if m 1 and n 2, the reaction is first order in A and second order in B. Unlike the first-order rate law, the second-order depends on two reactants and thus.
HALF LIFE OF SECOND ORDER REACTION FULL
The integral form of the equation was obtained from the differential form and the full integration can be found here. Referring to the generic rate law above, the reaction is m order with respect to A and n order with respect to B. The second-order rate law1 equation, specifically the integral form looks at the concentration of the reactants at a certain point in time. After 2 hours, a radioactive substance becomes (1/16) th of original amount. The Second order Half-Life calculator computes the half-life based on the temperature dependent reaction rate constant and the concentration of the substance. C) Each half-life is four times as long as the preceding one. B) Each half-life is twice as long as the preceding one. Half-lives can be used to determine the order of the reaction. The reaction orders in a rate law describe the mathematical dependence of the rate on reactant concentrations. d) The rate of a first order reaction does depend on reactant concentrations the rate of a second order reaction does not depend on reactant concentrations. 8) Which statement below regarding the half-life of a second-order reaction is true (you may want to do a second calculation using the info above) A) Each half-life is half as long as the preceding one. Reaction Half-Lives Reaction Orders and Reaction Half-life Pick a reactant concentration and read from the x axis the time it takes for it to half one, two and three times as shown in the figure below. The half-life of a reaction can be determined from a concentration-time graph.
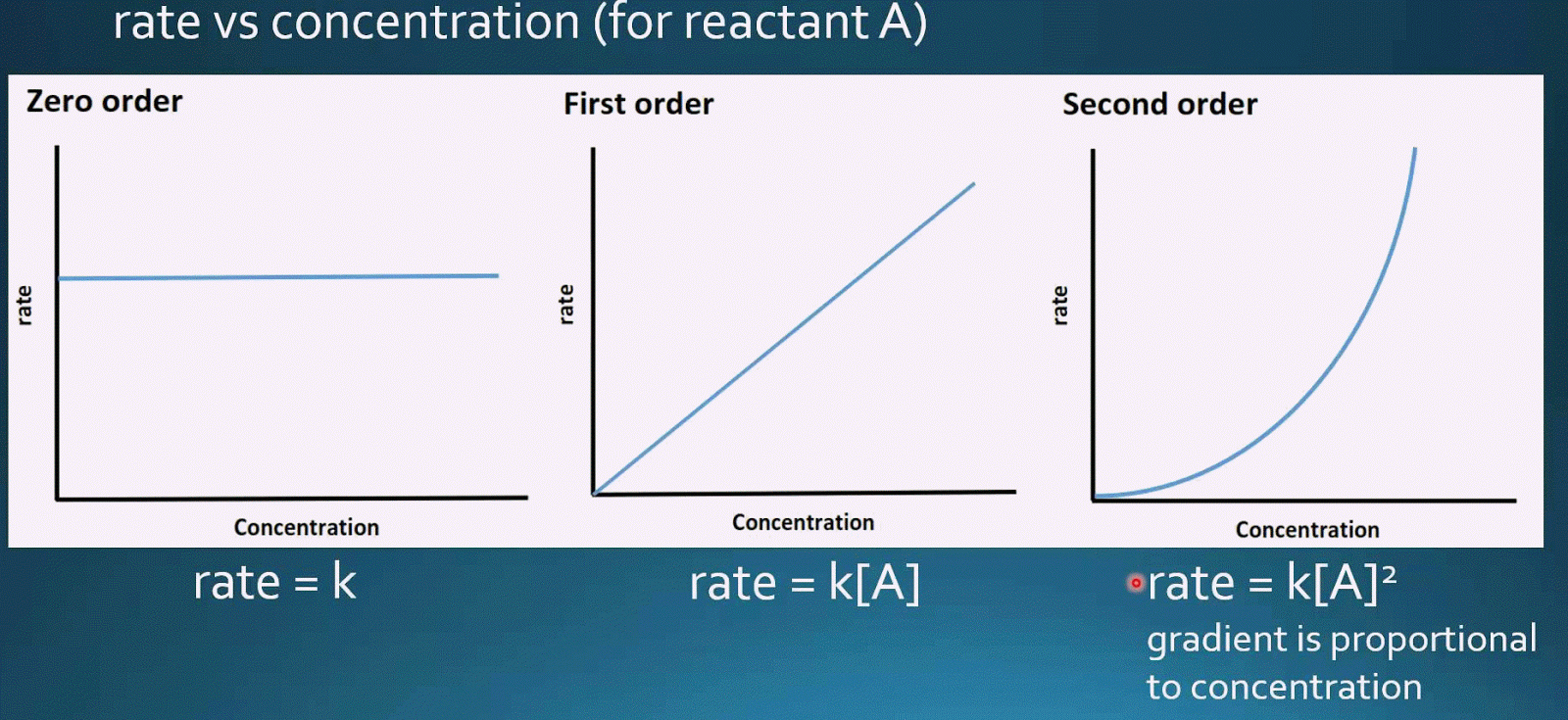
Determine the half-life of the reaction when the concentration of the reactant. What will be the half-life of the reaction when the concentration of the. b) The half-life for a zero-order reaction increases as. The half-life of a second-order reaction is 124 s when the concentration of the reactant at the start is 0.0122 M. The half-life of a second-order reaction is 120 s when the concentration of the reactant is 0.0100M. The half life (t1/2) of a reaction is the time taken for the concentration ofĪ reactant to half. A)The half-life for a reaction, aA Products, that is zero order in A increases with increasing A0. Unlike with first-order reactions, the rate constant of a second-order reaction cannot be calculated directly from the half-life unless the initial concentration is known.Rate Equations - Reaction Half Lives (A-Level Chemistry) Reaction Half-Lives Reaction Half Lives Consequently, we find the use of the half-life concept to be more complex for second-order reactions than for first-order reactions. And if this is a second order reaction, your graph should be a straight line. Therefore, the rate constant (k) of the reaction is 0.0625 min-1. We can derive the equation for calculating the half-life of a second order as follows: $$\frac $ is inversely proportional to the concentration of the reactant, and the half-life increases as the reaction proceeds because the concentration of reactant decreases. Zero-order reaction (with calculus) Kinetics of radioactive decay.


 0 kommentar(er)
0 kommentar(er)
